An atom is the basic unit that everything is made of. There are many different kinds of atoms, with different names, sizes, and weights*.
Atoms are very tiny. The largest one would be about 200,000 times smaller than the width of a human hair. Because they are so small, atoms can’t be seen without special tools.
So far scientists know about 118 different kinds of atoms. These kinds of atoms are called elements. You probably already know about some of them. For example, helium, neon, and gold are each made up of one single kind of atom. These are elements.
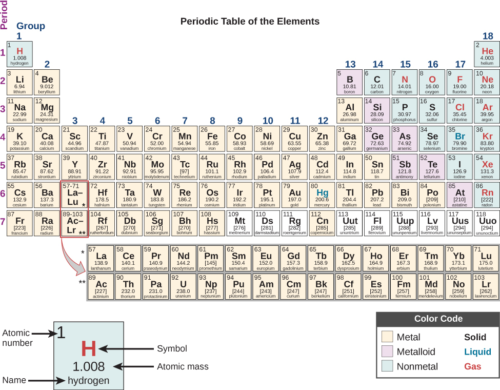
This is called the “Periodic Table of the Elements”.
(Source: OpenStax University Physics, via Wikimedia Commons.)
Molecules
Molecules are formed when atoms join together. For example, you might have heard someone call water “H2O”. That’s because two hydrogen(H) atoms and one oxygen(O) atom combine to make a water molecule.
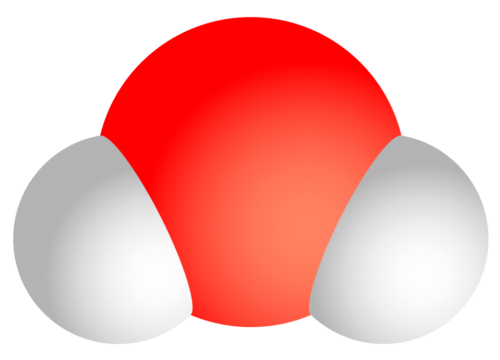
(Source: Wikimedia Commons.)
A molecule is the smallest amount of something you can get without breaking it down into something different. For example, if you took one water molecule and broke it down, you wouldn’t have water anymore. You’d have hydrogen and oxygen.
What makes up an atom?
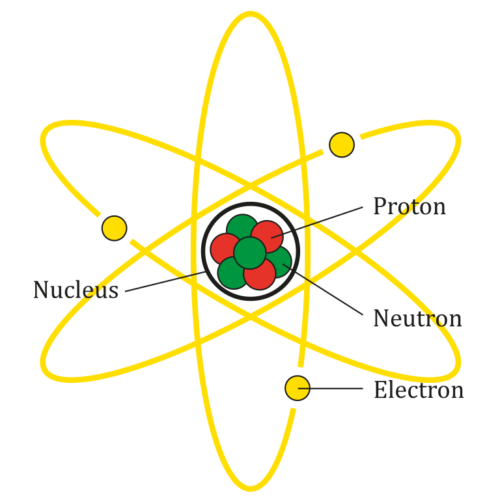
The diagram shows the parts of a Lithium atom. The atom does not actually look like this.
(Source: AG Caesar, from Wikimedia Commons.)
Every atom is made up of three kinds of smaller parts: protons (which are positively charged), neutrons (which have no charge) and electrons (which are negatively charged). Protons and neutrons are heavier, and stay in the middle of the atom. They are called the nucleus. They are surrounded by a cloud of moving electrons which are very light and are attracted to the positive charge of the nucleus.
Different atoms have different numbers of protons, neutrons, and electrons. Counting these will tell you what element an atom is. For example, a Lithium atom has 3 protons, 3 or 4 neutrons, and 3 electrons.
*Scientists usually prefer to say “mass” instead of “weight”.
